Identification of chemicals involved in host larvae recognition and acceptance by C. typhae
Foraging parasitoids cue on their host and host-habitat chemical signals in host recognition and selection processes. When different hosts are present in the same plant or field, the perception of specific volatiles and contact compounds emitted from the host itself enable the parasitoids both to differentiate between hosts and non-hosts and to estimate the health status of its host. Thus oral secretions from the feeding host, perceived by contact, can play a key role in such evaluation by the parasitoid.
Behavioral observations and biochemical approaches revealed that female parasitoids of the Cotesia flavipes species complex, including C. typhae, recognize their respective host species and oviposit in reaction to an α-amylase, a digestive enzyme present in the oral secretions of the host larvae (Bichanga et al. 2018a, 2018b). It has been further demonstrated that the conformation of the enzyme rather than its catalytic site as well as its substrate and its degradation product is responsible for host acceptance and oviposition mediation of Cotesia females.
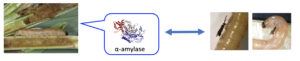
Legend :
- Lepidoptera stemborer larva found in its tunel filled with frass
- Digestive enzyme in the oral secretion of feeding larvae. Traces are present in the larval frass and on the host body.
- Frass extract deposited on a cotton ball (left) triggers antennal exam, as well as α-amylase alone ; when deposited on washed host body, the enzyme restores host acceptance for oviposition (right).
Cotesia male and female fertility and reproductive behaviour
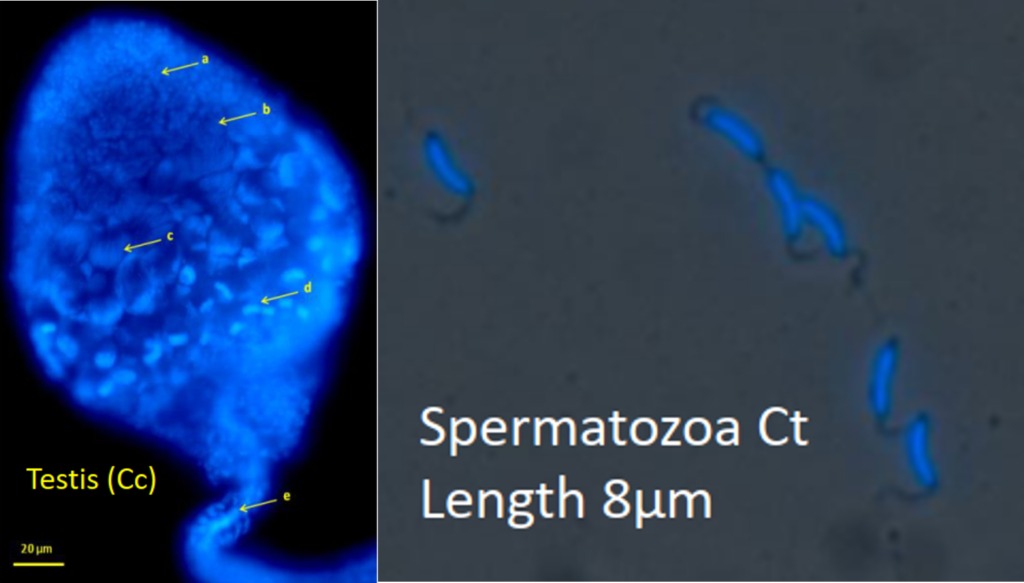
Knowing the reproductive potential of insect parasitoids is essential in biocontrol to adjust the number of released insects to the size of the pest population. This potential depends on the number of mature ovocytes and spermatozoas (initial fertility) and on the reproductive behaviour.
C. typhae females are pro-ovigenic, meaning that most eggs are mature upon emergence or soon after. A female produces about 200 ovocytes (Benoist et al. 2020).
Males also emerge with mature spermatozoas, but spermatogenesis continues for a few days after emergence. The peak is reached in three days, with about 22000 spermatozoas.
Adults mate upon emergence and can remate during their short life. In insects, after copulation spermatozoas are stored in a female reservoir called the spermatheca. Ovocytes fertilization occurs in the oviduct where the spermatheca opens. Like most Hymenoptera, C. typhae is haplo-diploid, meaning that non-fertilized (haploid) eggs will develop in males, and fertilized (diploid) eggs in females. When mating is successful, the progeny will contain a mix of brothers and sisters, with various sex-ratios. Non-mated females will give birth to sons almost exclusively (but see Capdevielle et al., 2022) .


Virulence and resistance: how it works and our projects
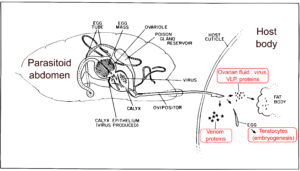
Endoparasitoids have to overcome the immune defenses of their host to develop successfully. Indeed, arthropod immune system is able to detect the presence of foreign bodies within the hemolymph (the insect “blood"), and to proceed to their destruction, either by phagocytosis (for small pathogens) or by encapsulation (for larger bodies, like parasitoid eggs). This is generally accompanied by the production of several defense molecules, such as anti-viral, anti-bacterial or cytotoxic compounds (one of them being the melanin, the characteristic black molecule often seen on wounded arthropods). This phenomenon is called “host resistance”.
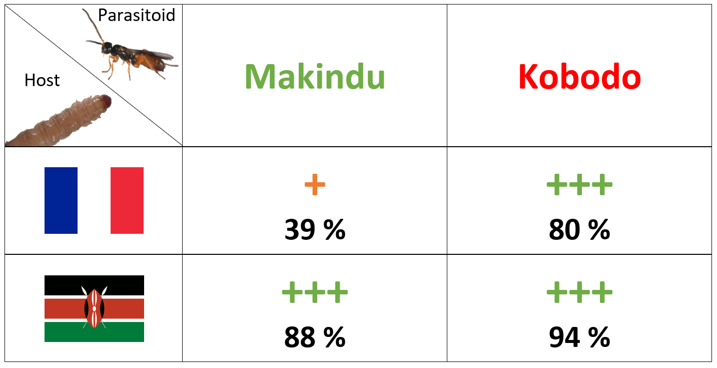
Kenyan strains of Cotesia typhae collected in two localities (Kobodo and Makindu) differ in their parasitism success when laying eggs in Sesamia nonagrioides larvae from a French population. Indeed, while both strains score a good parasitism success in the Kenyan host, the success of Makindu collapses by half on the French host, while that of Kobodo remains high.
There is a bilateral interaction undermining the parasitism success here. While the Makindu strain presents a deficient virulence, compared to the Kobodo one, the French host seems to possess resistance features compared to the Kenyan. Our project aims at determining which are the physiological and genetic components of parasitism success in this interaction. Virulence is partly genetically determined (Benoist et al. 2017) and a few DNA segments are involved in this trait (Benoist et al. 2020). They carry hundreds of candidate genes. A transcriptomic comparison will help us identify virulence genes in the parasitoid, and resistance genes in the host. Then, a comparison of the protein content between the two strains will guide us toward key venom proteins implied in the interaction.
Virulence and resistance: how symbiotic bracovirus of Cotesia interact with the host genome?
Bracoviruses (BVs) are domesticated viruses found in the genomes of some families of Braconidae parasitoid. They are composed of domesticated genes from a nudivirus, coding viral particles in which parasitoid DNA circles are packaged. During parasitism, Braconidae parasitoids, including C. typhae, inject these BV in their hosts during egg-laying. Infected host cells will produce viral proteins that will inhibit the host immune response against the parasitoid eggs. 27 proviral DNA segments were annotated in the genome of C. typhae, and 16 of the resulting DNA circles were shown to integrate massively in the chromosomes of various somatic tissues of the host (Muller et. Al. 2021).
In surviving Sesamia nonagrioides, chromosomal integrations of BV persist up to the adult stage. However, the transmission of these integrated BV to offspring has not yet been observed, suggesting either that host gametes are less targeted by BV than somatic cells or that gametes bearing BV integrations are nonfunctional (Muller et al. 2022).
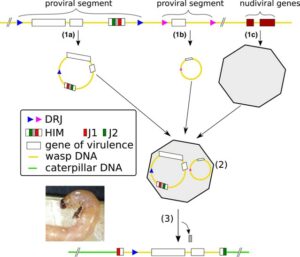
Structure of bracoviruses. In the parasitoid genome, (1a) illustrates a segment containing a Host-Integration-Motif (HIM), (1b) a segment devoid of HIM and (1c) a segment with genes of viral origin. White rectangles are virulence genes. In the wasp calyx, (1a) and (1b) form DNA circles thanks to their Direct Repeat Junctions (DRJ), whereas (1c) forms viral particles. DNA circles are packaged into viral particles (2), which are injected in the caterpillar host at the same time as the wasp eggs. In the host cells infected by the particles, the HIM‐containing segments can integrate in the chromosomes (3), and HIM locus called J1 and J2 delimit the integration, allowing to reveal such integration when sequencing the host genome. (Muller et al. 2022).
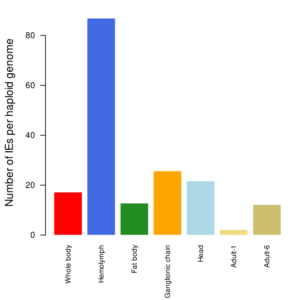
Estimated number of BV integration events (IEs) per haploid genome of S. nonagrioides.
The first 5 samples were sequenced 7 days post-parasitism (Muller et Al. 2021), whereas the two last samples correspond to the whole body of 2 surviving adults (Muller et Al. 2022).
Virulence and resistance: candidate virulence genes: quantitative genetic approach
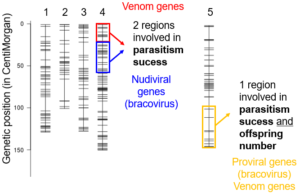
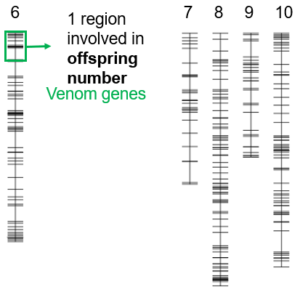
A cross experiment was conducted to identify genetic components explaining the difference in reproductive success between the two Cotesia typhae strains Kobodo and Makindu. The progeny of hybrid females were phenotypically characterized for 1) their ability to successfully parasitize a french population of Sesamia nonagrioides, 2) the number of offsprings produced by each female and 3) the ratio of males versus females among the offsprings. The same progenies were also genetically characterized with molecular markers differing between the two strains. Thanks to this genetic data, the markers were organized along the ten chromosomes of C. typhae to produce a genetic map. A test for cosegregation between phenotypic traits and genetic variant (either Kobodo or Makindu) was then performed along the chromosomes. This aproach allows to identify chromosomal regions involved in quantitative variation, called Quantitative Trait Loci (QTL). No QTL was found for sex ratio. For the two other traits, four QTL were described, one of them being linked to both parasitism success and offspring number (Benoist et al. 2020).
The molecular markers were localized in the reference genome of Cotesia typhae to list the genes annotated within each QTL region. Genes of specific interest were identified comprising venom genes and genes belonging to proviral or nudiviral segments of the bracovirus.
Quantitative Trait Loci (QTL) involved in reproductive success. Each vertical bar represents a chromosome of Cotesia typhae and each horizontal segment is a molecular maker. The four QTL identified are the color boxes. The genes of interest found in each QTL region are mentioned.
Sex determination in C. typhae
Hymenopterans are haplodiploid organisms: females are diploid and males are haploid. Arrhenotokous reproduction is believed to be the ancestral mode of reproduction in Hymenopterans. In this system, females are produced sexually and males are produced through parthenogenesis. Cotesia typhae uses this mode of reproduction, which means that mated females have a mixed offspring of males and females, whereas unmated females produce only male offspring.
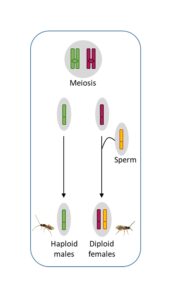
However, we discovered in the lab that sometimes unmated females produce a small number of females amongst the majority of male offspring. The production of females through parthenogenesis is called thelytoky. We noticed this phenomenon in lab strains as well as in a wild population, and we confirmed that parthenogenetically produced females are viable and fertile. With the use of genetic markers, we also found that even though daughters of mated females are mainly produced sexually, there is a small proportion of daughters that are produced through parthenogenesis (Capdevielle Dulac et al, 2022).
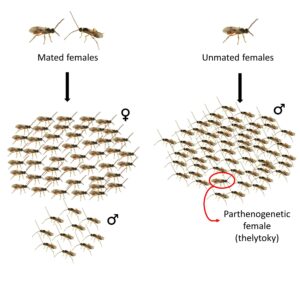
Thelytoky can operate either via a genetic process, or due to the presence of specific bacteria. Although thelytoky has been reported in many species, it is rare to observe it at a low frequency like in Cotesia typhae. Usually, populations are either completely arrhenotokous or completely thelytokous.
Sexual reproduction conveys a higher capacity to adaptation, thanks to recombination and its associated diversity. On the other hand, parthenogenesis can be advantageous in stable environments because it costs less energy. It would be interesting to know if the low frequency thelytoky we observe in Cotesia typhae is stable or transitory, as it could help understand how species evolve between sexual and parthenogenetic reproduction modes.